Usuarios del Club TRPlane
Calificar los síntomas en una escala numérica parece fácil, pero puede ser más difícil de lo que parece. ¿Cuánto te dolió cuando te golpeaste el dedo del pie? ¿Fue un dos de 10? ¿Un cuatro? ¿Con qué frecuencia te despiertas por la noche? ¿Dos, tres veces? ¿Regularmente? ¿Casi nunca?
Estas escalas imprecisas y autoinformadas se pueden encontrar en toda la medicina, incluso en relación con enfermedades en las que lo que está en juego es más alto que una uña magullada. Koneksa, fundada en 2013, está orientada en descubrir y validar biomarcadores clínicos. Ese proceso comienza buscando convertir esas escalas analógicas en digitales y mejorarlas en el proceso.
Koneksa ha desarrollado software que funciona con una variedad de dispositivos, desde espirómetros portátiles hasta iPhones y Apple Watch. Estos dispositivos capturan datos, de los cuales el software obtiene información clínicamente valiosa y la organiza para su revisión por parte de las compañías farmacéuticas o los proveedores de atención médica que realizan ensayos clínicos.
Gracias a varios estudios y al trabajo con ensayos clínicos en más de 700 localizaciones, Koneksa anuncia un aumento de la Serie C de 45 millones de dólares. La compañía ha sido comparativamente modesta hasta ahora, recaudando 4 millones en la Serie A y fondos iniciales, y una Serie B de 16 millones.
Esta ronda será parte de un gran impulso para marcar el comienzo de lo que el CMO John Wagner llama un «punto de inflexión» en el mundo de los biomarcadores digitales. (Wagner llega a Koneksa después de trabajar en Cynga, Merck, Takeda y Foresite Capital. También editó la revista Clinical and Translational Science).
“Es bien sabido que cuantas más medidas tomes, más precisión obtendrás y más certeza puede tener un ensayo clínico. Lo que hace el análisis más rápido y más eficiente”, dijo Wagner. “Me gusta pensar en ello como la diferencia entre una foto fija y un video. Una foto fija es genial, pero un video puede contar toda la historia. Así es como pensamos en los biomarcadores digitales: cuentan toda la historia en comparación con los biomarcadores tradicionales”.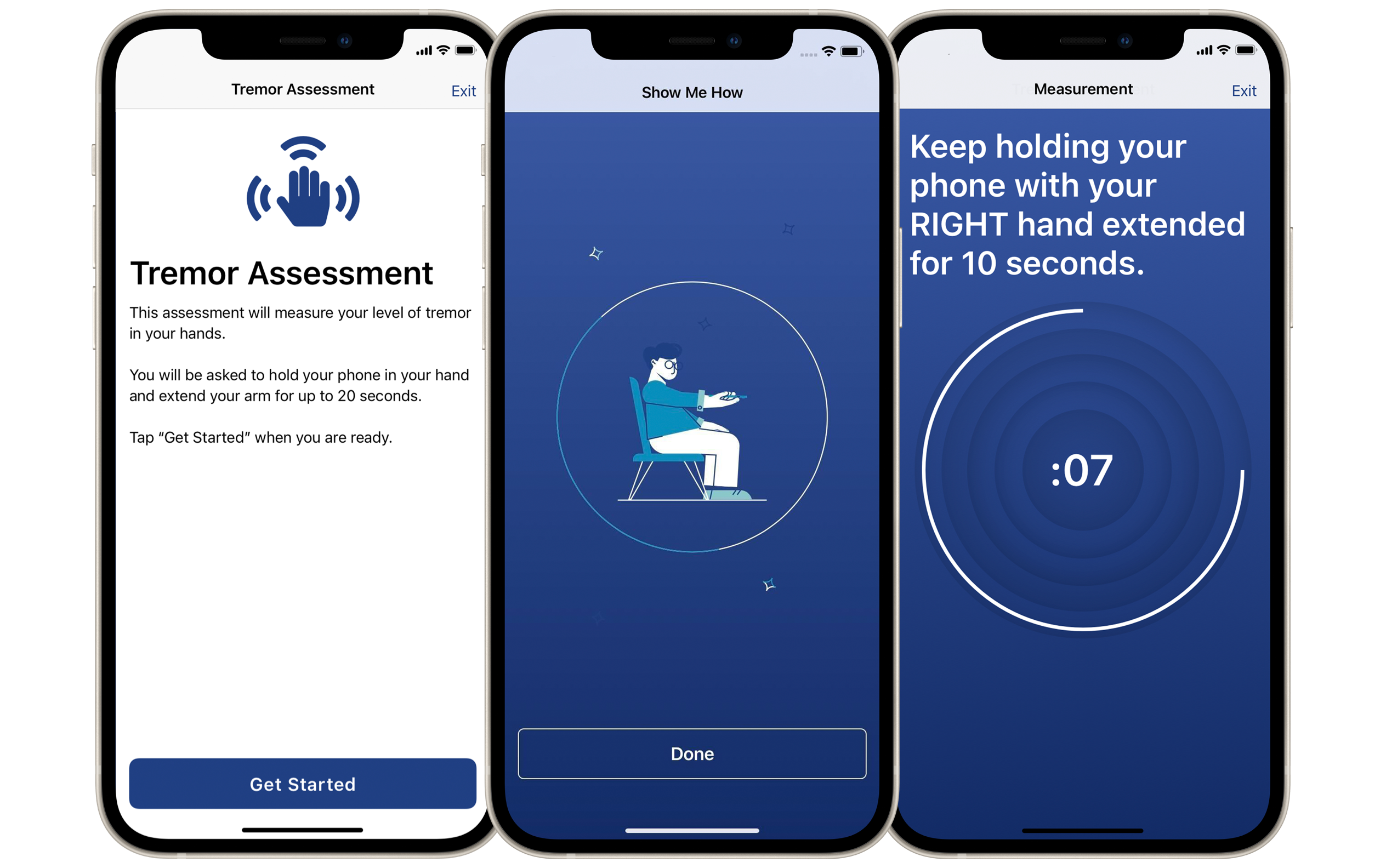
Herramienta de evaluación de temblores de Koneksa para el Parkinson.
¿Cómo Koneksa convierte las escalas analógicas en digitales? El Parkinson ofrece un claro ejemplo. Una común, pero imperfecto, índice utilizado para evaluar a los pacientes de Parkinson se denomina Escala unificada de Calificación de la Enfermedad de Parkinson. Es un examen de varias partes que combina cuestionarios sobre el estado de ánimo, la vida diaria y los síntomas, con una evaluación clínica, como pruebas de movimiento.
Para cuantificar un temblor, un médico podría pedirle a un paciente que estire un brazo, y voltea la palma de su mano hacia arriba y hacia abajo lo más rápido que pueda. Mientras tanto, el médico observa cambios sutiles en la velocidad o la forma de ese movimiento. El enfoque de Koneksa, en cambio, hace que el paciente sostenga su teléfono y realice el mismo movimiento. El acelerómetro y el giroscopio del teléfono registran esos cambios y transmiten datos a la plataforma de la empresa.
Tras ello, los algoritmos de la compañía «valoran» ese temblor, de forma similar a cómo un médico podría asignar una puntuación después de un examen.
“El potencial de estos sensores para dar es mucho más granular y, uso esta palabra con cuidado, una lectura más objetiva del temblor de un hombre: hay un tremendo potencial en lo que nuestra tecnología puede hacer”, dijo el CEO Chris Benko a TechCrunch.
La idea de modernizar las técnicas analógicas de monitorización de pacientes con tecnología ya ha atraído a muchas empresas. Koneksa está mejorando ese servicio, pero no tiene la vista puesta solo en el software. Se trata de probar toda la tesis de la fiabilidad de los biomarcadores digitales, si no la mejora de los mismos en el proceso.
Un teléfono puede detectar pequeños movimientos de la mano que un médico podría pasar por alto. Pero, ¿esos movimientos de manos realmente representan temblores? Y, ¿estas pruebas digitalizadas realmente les dicen a los médicos algo sobre cómo evolucionarán los pacientes en el futuro?
Para conocer las respuestas a esas preguntas, los biomarcadores digitales deben validarse clínicamente. Sin esa validación, tales herramientas son, en última instancia, inútiles en los ensayos clínicos.
“Queríamos centrarnos en los problemas de control que son importantes para comercializar nuevas terapias para los pacientes, y luego descubrir cómo realizar la validación científica que muestre que estas herramientas tecnológicas son mejores o equivalentes a lo que existe. Tenemos que hacerlo al nivel de las principales compañías farmacéuticas del mundo”, dijo Benko.
Esa validación es el enfoque actual de Koneksa. La empresa ha podido demostrar que las medidas tomadas en el hogar se correlacionan con las pruebas realizadas en una clínica.
Un estudio con 12 pacientes con asma, por ejemplo, mostró que las lecturas de un espirómetro en el hogar eran comparables a las realizadas en una clínica. Los pacientes cometieron algunos errores, señaló Benko, pero debido a que pudieron realizar la prueba con más frecuencia, los investigadores terminaron con muchos más datos con los que trabajar.
Un ensayo clínico típico de asma, señala el estudio, necesitaría inscribir a unos 100 pacientes para obtener un resultado significativo. Usando las pruebas en el hogar, los autores de este informe sugieren que podrían obtener los mismos resultados con solo 18 personas. “Eso es lo que es económicamente interesante para la compañía farmacéutica”, dijo Benko. «Pero para los pacientes, simplemente dicen que es más conveniente y es más real».
La compañía también ha podido presentar un caso convincente de que los datos de los pacientes en el hogar pueden brindar información clínicamente significativa. Por ejemplo, la compañía realizó un estudio en 66 pacientes con cáncer cerebral y garganta, donde usaron dispositivos portátiles para medir el número de pasos diarios.
Por cada 1000 pasos caminados, el estudio encontró una reducción del 26% en el riesgo de hospitalización de los pacientes. Sin embargo, como señaló Benko, fue su actividad los fines de semana (es decir, el movimiento que eligieron los propios pacientes) lo que reveló esta estadística, no los horarios de movimiento debido a las responsabilidades de los días laborales.
Ese estudio se presentó en la reunión de la Sociedad Estadounidense de Oncología Clínica de 2021, pero no llegó a publicarse en una revista.
Aun así, quien decide en última instancia sobre la validez clínica, al menos cuando se trata de desarrollar fármacos, en el caso de Estados Unidos, es la FDA. El siguiente paso de Koneksa será demostrar que estos biomarcadores son lo suficientemente fiables como para integrarlos en el proceso de toma de decisiones regulatorias.
“Lo que estamos preparando en este momento son estudios de propiedad de Koneksa o de colaboración de Koneksa con académicos para impulsar la validación clínica”, dijo Wagner.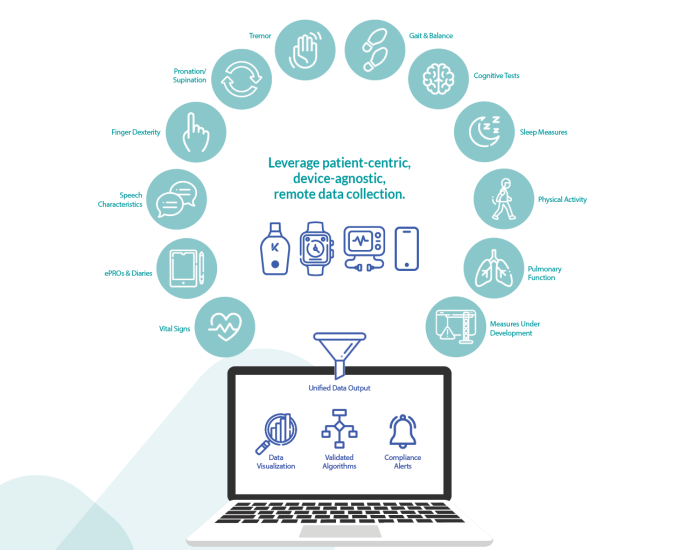
Gama completa de medidas de Koneksa
En cuanto al capital conseguido en la Serie C, Koneksa tiene dos objetivos.
El primero es agregar las evidencias que respaldas su flujo de biomarcadores digitales. En el pasado, la empresa ha trabajado junto con sus socios farmacéuticos para buscar biomarcadores digitales y validarlos. Obtener la validación antes de estas asociaciones permitirá a Koneksa poner en marcha nuevos programas mucho más rápido.
La segunda pieza es un lanzamiento de lo que Benko llama una plataforma de «autoservicio». Esta plataforma de autoservicio permite a los nuevos socios combinar datos de múltiples dispositivos y dispositivos portátiles en el mismo lugar y organizarlos en un espacio común.
“Reconocemos que la capacidad del software para integrar ese caos de datos del dispositivo en un entorno de ensayo clínico son únicos. No hay otro activo como ese en el mercado. Entonces, ¿por qué deberíamos controlarlo para cada uso posible? Queremos poder abrirlo para decir: ‘podemos licenciarte esta plataforma'».
Aunque estos dos objetivos son operacionalmente distintos, tienen un objetivo similar. Koneksa busca convertir su plataforma y biomarcadores digitales en una solución llave en mano.
Esta ronda fue liderada por AyurMaya, fondo administrado por Matrix Capital Management, con participación de Takeda Ventures y Velosity Capital. Los inversores anteriores McKesson Ventures, Merck Global Health Innovation Fund, Novartis (dRx Capital), Spring Mountain Capital y Waterline Ventures también participaron en la ronda.

